Mössbauer spectrometry
LEA distributes Mössbauer sources from RITVERC for more than 15 years. But what is Mössbauer spectrometry?
-
Zoom on ... Mössbauer sourcesDownload
Mössbauer spectrometry is a non-destructive nuclear resonance method for studying the valence states of atoms, their chemical bonds as well as their coordination within solid phases.
This technique can be used for about 40 isotopes (the most commonly studied being 57Fe, 119Sn, 119Sb, 170Dy and 197Au), in areas ranging from atomic physics to metallurgy and physics and solid-state chemistry, nanoscience, condensed matter physics, archeology, mineralogy (the Spirit and Opportunity rovers have bring a Mössbauer spectrometer for the study of Martian soil).
Mössbauer spectrometry can be applied to all kinds of solid materials (crystalline systems, amorphous, quasicrystalline, nanocrystalline, metallic alloys, insulators, conductors, polymers) and nano structures (nanostructure powders, nanoparticles).
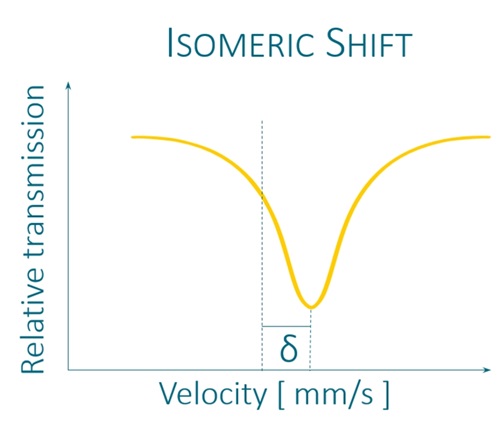
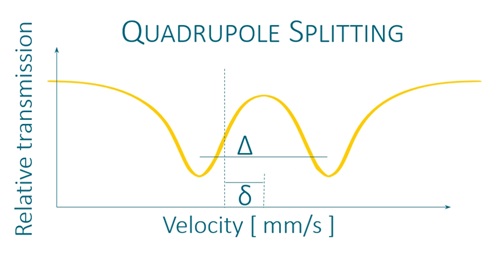
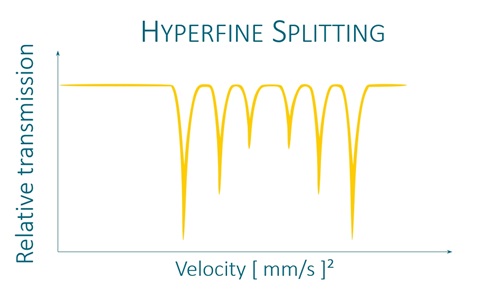
It uses a radioactive source to excite the studied isotopes and to observe the perturbations of their nuclear energy levels by the measurement of 3 parameters: the isomeric shift, the quadrupole separation and the hyperfine field.*
* source : article 103 of GFSM
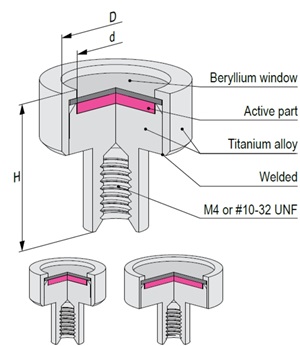
The active part of Mössbauer source is prepared by electrodepositing high purity carrier-free 57Co onto a thin metal backing (matrix) followed by controlled annealing process. Standard matrix is rhodium or chromium. Other matrices are available on request. All sources are carefully tested on certified equipment. Each source is supported by a Test Report with measured values of spectra parameters.
Recommended working life: 10 years
Capsule Type | D x H [mm] | d [mm] | Line Width [mm/s] | ISO Classification | Temperature range [K] | Nominal Activity* | Code | ||
mCi | MBq | ||||||||
Rhodium matrix | |||||||||
1 | 11.2 x 13 | 8 | 0.11 – 0.13 | C54243 | 4.2 – 700 | 5 10 25 50 100 | 185 370 925 1850 3700 | MCo7.111 MCo7.112 MCo7.113 MCo7.114 MCo7.115 | |
2 | 14 x 14 | 8 | 0.11 – 0.13 | C54243 | 4.2 – 700 | 5 10 25 50 100 | 185 370 925 1850 3700 | MCo7.121 MCo7.122 MCo7.123 MCo7.124 MCo7.125 | |
6 | 6 x 13 | 4 | 0.11 – 0.15 | C54243 | 4.2 – 700 | 5 10 25 50 100 | 185 370 925 1850 3700 | MCo7.161 MCo7.162 MCo7.163 MCo7.164 MCo7.165 | |
9 | 4 x 14 | 1 | 0.13 – 0.15 | C34243 | 220 – 450 | 5 10 | 185 370 | MCo7.191 MCo7.192 | |
10 | 6 x 17 | 1 | 0.13 – 0.15 | C34243 | 220 – 450 | 5 10 | 185 370 | MCo7.1101 MCo7.1102 | |
Chromium matrix | |||||||||
1 | 11.2 x 13 | 8 | 0.13 – 0.16 | C5443 | 4.2 – 700 | 5 10 25 50 | 185 370 925 1850 | MCo7.511 MCo7.512 MCo.513 MCo.514 | |
2 | 14 x 14 | 8 | 0.13 – 0.16 | C54243 | 4.2 – 700 | 5 10 25 50 | 185 370 925 1850 | MCo7.521 MCo7.522 MCo7.523 MCo7.524 | |
* Tolerance: -5% to +10% | 14.41 keV photon emission efficiency ≥ 75% | Recoilless fraction : 0.75
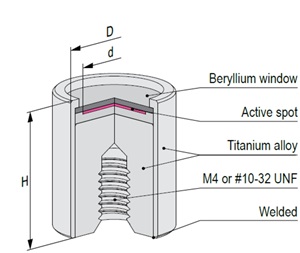
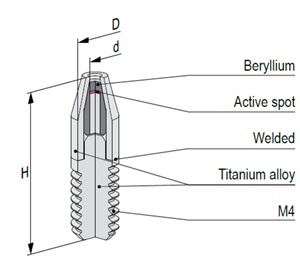
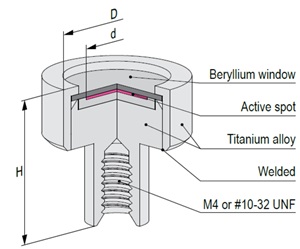
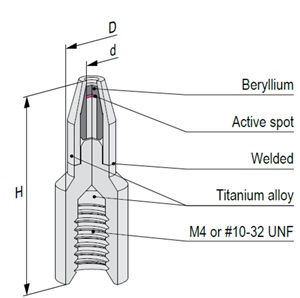
Mössbauer source active part is based on calcium stannate [CaSnO3] matrix synthesized from high specific activity (>300 mCi/g) 119mSn radionuclide. All sources are carefully tested on certified equipment. Each source is supported by a Test Report with measured values of spectra parameters.
Recommended working life: 10 years
Capsule Type | D x H [mm] | d [mm] | Line Width [mm/s] | ISO Classification | Temperature range [K] | Nominal Activity* | Code | ||
mCi | MBq | ||||||||
1 | 11.2 x 13 | 10 | 0.38 – 0.54 | C54243 | 4.2 – 700 | 2 5 10 | 74 185 370 | MSn9.211 MSn9.212 MSn9.213 | |
2 | 14 x 14 | 10 | 0.38 – 0.54 | C54243 | 4.2 – 700 | 2 5 10 15 | 74 185 370 555 | MSn9.221 MSn9.222 MSn9.223 MSn9.224 | |
3 | 18 x 14 | 15 | 0.38 – 0.54 | C54243 | 4.2 – 700 | 10 15 20 | 370 555 740 | MSn9.233 MSn9.234 MSn9.235 | |
6 | 6 x 13 | 5 | 0.45 – 0.54 | C54243 | 4.2 – 700 | 2 5 | 74 185 | MSn9.261 MSn9.262 | |
* Tolerance: -5% to +10% | 23.87 keV photon emission efficiency ≥ 75% | Recoilless fraction : 0.5
8cc4e0a7f16c4a25acf77d7e6a23b98b.tmb-300.jpg?sfvrsn=a8aa09_1)
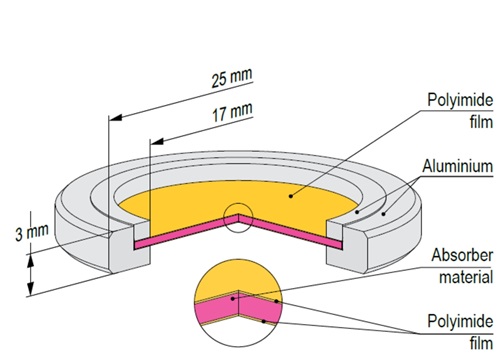
Mössbauer effect reference absorbers contain chemical substances synthesized with either enriched ⁵⁷Fe (>95%) or natural iron. The substances are uniformly dispersed in polyethylene dics of 1 mm thickness and 20 mm diameter, placed between two polyimide films in aluminium holders.
K4Fe(CN)6 × 3H2O and K2MgFe(CN)6 exhibit unsplit narrow line in Mössbauer absorption spectra. FeC2O4 × 2H2O exhibits quadrupole splitting, leading to two narrow lines in Mössbauer absorption spectrum. αFe and Fe2O3 exhibit magnetic hyperfine splitting, leading to six narrow lines in Mössbauer absorption spectra. All reference absorbers are carefully tested on certified equipment.
Each absorber is supported by a Test Report with measured values of Mössbauer spectra parameters.
Description | Thickness [mg 57Fe/cm²] | Code | |
Enriched iron reference absorbers | |||
K2MgFe(CN)6 | 0.25 – 1 | MRA.1.1.X* | |
FeC2O4 x 2H2O | 0.5 – 1 | MRA.1.2.X* | |
Fe2O3 | 1 – 2 | MRA.1.3.X* | |
α-Fe foil | 3 µm | MRA.1.6 | |
Natural iron reference absorbers | |||
FeC2O4 x 2H2O | 0.13 – 0.25 | MRA.2.2.X* | |
Fe2O3 | 0.13 – 0.25 | MRA.2.3.X* | |
K4Fe(CN)6 x 3H2O | 0.13 – 1 | MRA.2.4.X* | |
α-Fe foil | 30 µm | MRA.2.6 | |
* « X » represents the thickness of the absorber in mg 57Fe/cm². Available values of X = { 1 ; 2 ; 3 ; 4 ; 5 }, respectively for { 0.13 ; 0.25 ; 0.5 ; 1 ; 2 } mg 57Fe/cm²
