Nuclear medecine the hope against cancer
When nuclear science meets biotechnology

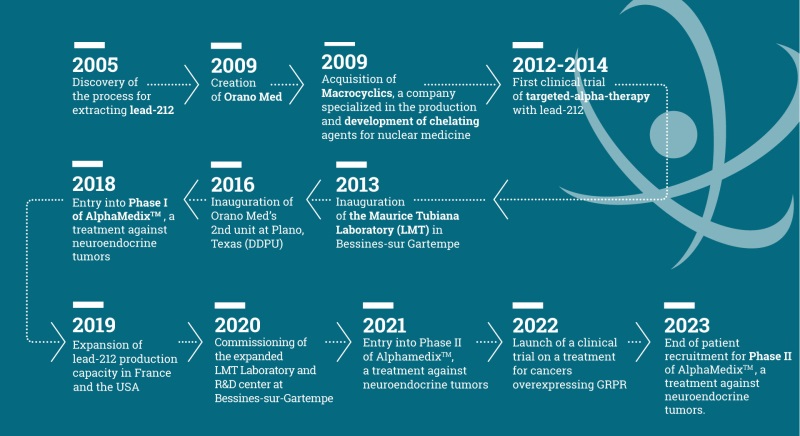
In the early 2000s, the Orano group looked at opportunities to use material derived
from its core nuclear energy activities. Nuclear medicine and Radioligand Therapy quickly appeared very promising. Lead-212 was identified as offering tangible scientific prospects, and Orano had the necessary know-how to meet the
challenges involved in its procurement.
With sufficient resources to produce Lead-212 in larger quantities, the project soon emerged to demonstrate the feasibility of extracting and purifying this isotope. Successful results followed by conclusive preclinical studies with the National Cancer Institute in the US led to the creation of Orano Med in 2009.
The properties of Lead-212

Lead-212 is a very rare radioactive alpha-isotope that descends from Thorium. Orano’s expertise in cutting-edge nuclear technologies has made it possible to develop a unique process for the extraction and purification of Lead-212. This rare metal is the subject of much research and is used in promising new treatments againt cancer, called Targeted Alpha Therapy (TAT).
Lead-212 has all the biological qualities required for application in radioligand therapies:
- Its half-life of under 11 hours facilitates the management of radioactive waste and effluents and allows treatments to be administered on an outpatient basis, placing less constraints on hospitals;
- It only has a single alpha emission in its decay chain, limiting the circulation of free radioactive isotopes (not chelated to the vector) and thus the toxicity to healthy organs;
- It has a particularly stable chelating agent, allowing effective targeting of cancer cells;
- Another isotope of lead, Lead-203, is an imageable gamma emitter, allowing the development of theranostic approaches with 203Pb / 212Pb.
Hopes for Targeted Alpha Therapy

Targeted Alpha Therapy (TAT) is an innovative technology
combining 212Pb with diverse biological vectors (peptides, antibodies) targeting or binding to different specific cancer receptors or antigens. TAT has the property to selectively recognize and destroy cancer cells, limiting the impact on surrounding healthy cells.
This therapeutic approach is fueling the hope of the international medical community to move towards less toxic and more effective treatments for patients with limited therapeutic solutions.
Superiority of targeted alpha therapy in the fight against cancer

Two types of isotopes can be used in radioligand therapies: emitters of alpha or beta
radiation. Currently, only beta therapies are commercially available. However, alpha particles have two key benefits for applications in oncology:
1 - Improved biological efficacy
Alpha decay consists of the emission of a helium nucleus (alpha particle) together with linear energy transfer which is 100 times higher than that of beta radiation. The alpha radiation thus causes irreparable double-strand breaks in the DNA of cells in immediate proximity to the emission while beta radiation has more of a tendency to cause single-strand breaks.
Fewer than five alpha particles are needed to kill a cancer cell, versus thousands of chemotherapy molecules.
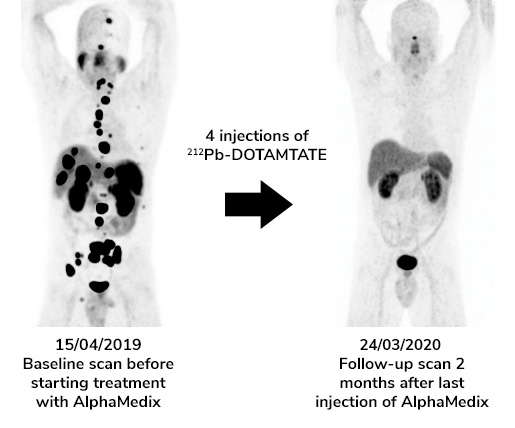
Patient with metastatic neuroendocrine tumors included in the phase 1 clinical trial of AlphaMedix (212Pb-DOTAMTATE), a drug currently being developed by Orano Med and RadioMedic.
2 - Toxicity limited thanks to short range of emission
The alpha particles only travel over a very limited distance into the tissues: only 2 to 5 cell layers (compared to more than 50 with beta radiation). They thus deposit a very large amount of energy over a very short distance. This results in increased efficacy on the cancer cells while limiting toxicity to nearby healthy cells.
More information on www.oranomed.com