All about radioactivity
What is radioactivity?
Radioactive radiation
As they decay, radioelements emit radiation. This radiation, which can be of different kinds, is classified according to its ability to penetrate matter.

The phenomenon of ionization is the mechanism by which radioactivity acts on matter. Alpha, beta, gamma and X-rays disrupt the organization of living matter. Atoms placed in their path can lose one or more electrons. These atoms are then transformed into "ions" which, as they are electrically charged, will, in turn, disrupt the organization of the molecules or cells of which they are the component parts. This is why radioactive radiation is said to be "ionizing".
What is the difference between natural and artificial radioactivity ?
When the Earth was first formed, about 5 billion years ago, matter was made up of both radioactive and stable elements. Since then, the radioactivity has continued to decrease as many radioactive atoms have been transformed into stable elements. Some continue to change while others are still forming.
2/3 of the radioactivity to which humans are exposed is natural
1/3 of the radioactivity to which humans are exposed is artificial
Where does natural radioactivity come from?
Natural radioactivity comes mainly from four sources :
1° Cosmic radiation
 This originates from the sun and space. The strength of cosmic radiation increases with altitude: it doubles every 1,500 m. When traveling by plane at an altitude of 26,000 feet, the dose received is almost 100 times greater than that at sea level. Exposure thus rises from 0.5 mSv (millisievert) per person per year at sea level to around 2 mSv/year at ground level, to 50 mSv/year at altitude, to 500 mSv/year in orbit and to 1,000 mSv/year in space.
This originates from the sun and space. The strength of cosmic radiation increases with altitude: it doubles every 1,500 m. When traveling by plane at an altitude of 26,000 feet, the dose received is almost 100 times greater than that at sea level. Exposure thus rises from 0.5 mSv (millisievert) per person per year at sea level to around 2 mSv/year at ground level, to 50 mSv/year at altitude, to 500 mSv/year in orbit and to 1,000 mSv/year in space.
The highest cosmic ray dose rates are received by cosmonauts during space flights (1 mSv/day). For example, Thomas Pesquet received around 200 mSv during his mission on the International Space Station.
2° Ambient air
 The air gives off radon fumes; radon is a radioactive gas that results from the decay of uranium in the earth's crust. The average equivalent dose in French homes is 1.43 mSv per person per year. It varies, depending on the nature of the ground and the methods of construction and ventilation.
The air gives off radon fumes; radon is a radioactive gas that results from the decay of uranium in the earth's crust. The average equivalent dose in French homes is 1.43 mSv per person per year. It varies, depending on the nature of the ground and the methods of construction and ventilation.
3° Telluric radiation
 This is emitted by many radioactive elements present in the earth's crust, such as uranium and thorium. It varies according to the type of ground and thus changes from one region to another. Exposure to telluric radiation is, on average, 0.62 mSv per person per year in France.
This is emitted by many radioactive elements present in the earth's crust, such as uranium and thorium. It varies according to the type of ground and thus changes from one region to another. Exposure to telluric radiation is, on average, 0.62 mSv per person per year in France.
4° Food and drink
 Both contain radioactive elements which, after ingestion, attach themselves to organic tissues and bones. These are elements that are essential for life and have radioactive isotopes such as potassium 40 or carbon 14. Internal irradiation is, on average, 0.55 mSv per person per year.
Both contain radioactive elements which, after ingestion, attach themselves to organic tissues and bones. These are elements that are essential for life and have radioactive isotopes such as potassium 40 or carbon 14. Internal irradiation is, on average, 0.55 mSv per person per year.
Source: IRSN
Natural radioactivity has no detectable effect: it does not cause visible health damage and the body incorporates it as a natural component of the biological process.
Where does artificial radiation come from?
There are many applications of ionizing radiation.
1° Medical irradiation
 This is the source of the greatest exposure, thanks to the development of nuclear medicine, scanning and radiography. In the most industrialized countries, a person receives an equivalent dose of 1.8 mSv every year. The global average is 0.6 mSv per person per year.
This is the source of the greatest exposure, thanks to the development of nuclear medicine, scanning and radiography. In the most industrialized countries, a person receives an equivalent dose of 1.8 mSv every year. The global average is 0.6 mSv per person per year.
2° Technical and industrial applications
 They, too, constitute a source of radioactivity although their induced dose is negligible. Extractive mining, atmospheric fallout from military tests or, more daily, exposure to radiation emitted by televisions or computer screens, lead to an equivalent dose of 0.1 mSv per person per year. All the radiation generated by the nuclear power industry accounts for less than 0.01 mSv per person per year.
They, too, constitute a source of radioactivity although their induced dose is negligible. Extractive mining, atmospheric fallout from military tests or, more daily, exposure to radiation emitted by televisions or computer screens, lead to an equivalent dose of 0.1 mSv per person per year. All the radiation generated by the nuclear power industry accounts for less than 0.01 mSv per person per year.
How is radioactivity measured?
Radioactivity is a quantifiable phenomenon. There are three international units of measurement.
Each relates to data of a different type:
 Radioactive activity is measured in becquerel (Bq). It quantifies the number of radioactive nucleus decays that occur every second in a sample.
For example, around 9,000 atoms in the body of a 70 kg person disintegrate every second, so their activity is 9,000 Bq.
The old unit is the curie, equivalent to 37 billion becquerels.
Radioactive activity is measured in becquerel (Bq). It quantifies the number of radioactive nucleus decays that occur every second in a sample.
For example, around 9,000 atoms in the body of a 70 kg person disintegrate every second, so their activity is 9,000 Bq.
The old unit is the curie, equivalent to 37 billion becquerels.
 The amount of radiation absorbed by an organism or an object exposed to radiation is measured in gray (Gy).
The amount of radiation absorbed by an organism or an object exposed to radiation is measured in gray (Gy).
It is a measure of energy, representing 1 joule per kilogram of matter.
The gray replaced the rad (1/100th of a gray) in 1986.
 The biological effects of radiation on an exposed organism are measured in sievert (Sv). It is a radiation protection unit. It is expressed in "equivalent dose" and takes into account the characteristics of the radiation and of the irradiated organ.
The sievert replaced the rem (1/100th of a Sv) in 1986. The millisievert (mSv), or one thousandth of a sievert, is used very frequently.
The biological effects of radiation on an exposed organism are measured in sievert (Sv). It is a radiation protection unit. It is expressed in "equivalent dose" and takes into account the characteristics of the radiation and of the irradiated organ.
The sievert replaced the rem (1/100th of a Sv) in 1986. The millisievert (mSv), or one thousandth of a sievert, is used very frequently.
Radioactivity is detected and measured using equipment (Geiger counters, ionization chambers, scintillators) or devices (photographic films) that use the properties of radiation. These measurements achieve very high precision.

What are the effects of radioactivity on humans ?
The effects of radiation on the body vary greatly depending on the dose received, the time and method of exposure and the radioelement involved. Knowledge of the effects caused by radioactivity comes from the analysis of real cases of irradiation on people accidentally exposed or medically treated, or from epidemiological surveys on highly exposed populations (survivors of Hiroshima and Nagasaki for example) and of experimental studies. A scale of the risks associated with radiation exposure was thus drawn up.The early effects of ionizing radiation
They can be observed only after exposure to high doses of radiation and above a certain threshold. Depending on the dose received they vary from a transient change in the blood count without clinical signs (from 1 gray) to a lethal dose beyond any therapeutic options (above 15 gray).
The late effects of ionizing radiation
In particular, radiation acts on DNA molecules and can lead to the appearance of delayed pathological effects (cancer, leukemia, genetic alterations). These effects are random, that is, they do not appear systematically. It is usually considered that the probability of their occurring is proportional to the delivered dose of radiation (the lower the dose, the lower the probability of developing cancer).
Based on this approach, the official French radiation protection authorities have, as a precautionary measure, set very low limit levels for exposure to artificial radioactivity : 1 mSv/year for the public and 20 mSv/year for professionals working in the nuclear power industry. These limits are, respectively, 1,000 and 50 times lower than the doses leading to the first observable signs of early pathology.
How does one protect oneself from radioactivity?
Three types of protection
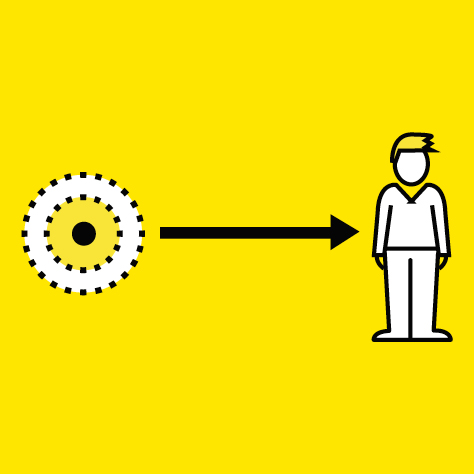 The primary safety measure is the distance between the organism and the radioactive source.
The primary safety measure is the distance between the organism and the radioactive source.
A security zone is thus defined around the exposed sites and all manipulations are carried out remotely within that zone.
 The duration of exposure to radiation is monitored in exposed areas.
The duration of exposure to radiation is monitored in exposed areas.
The harmfulness of the radiation depends on the dose received, which increases the longer the exposure to the radiation.
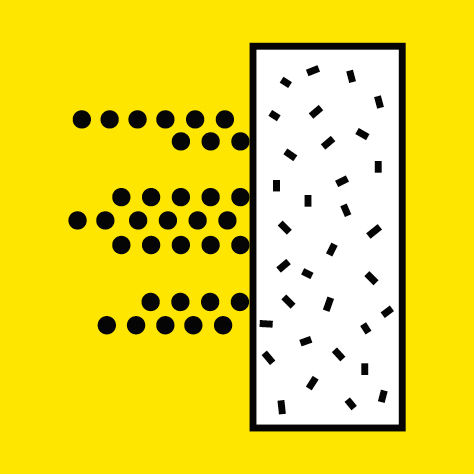 Protective screens made of lead, metal or concrete and of suitable thickness stop the radiation.
Protective screens made of lead, metal or concrete and of suitable thickness stop the radiation.
A few meters of water also provide effective protection. Wearing protective suits insulates professionals from the risk of contamination by unsealed radioactive sources.

Strict regulations
The aim of radiation protection is to prevent anyone from beingexposed to excessive doses of radioactivity.
Dose limits
They were intentionally set at very low rates in order to be safety limits and not danger limits. They concern both the public and professionals working in the sectors where nuclear radiation is present (hospitals, nuclear power plants, etc.). The doses delivered are monitored and measured systematically. Personal dosimeters make it possible to determine the dose received by each person working in a high-risk environment. Measurements of environmental radioactivity levels are made around facilities where radioactive products are handled and the doses received by the public are measured.
- 1 mSv/year: regulatory dose limit in France for public exposure to artificial radioactivity
- 20 mSv/year: regulatory dose limit for professionals working in the nuclear power industry
- 0.8 mSv/year: average dose in 2019 for Orano employees arising from exposure to ionizing radiation
The International Commission on Radiological Protection (ICRP) or the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) are responsible for issuing recommendations or ensuring the strict application of regulations and compliance with protection standards. In France, the Nuclear Safety Authority (Autorité de sûreté nucléaire -ASN) and the IRSN supervise and monitor nuclear and radiological risks.
The Institute for Radiation Protection and Nuclear Safety (l'Institut de Radioprotection et de Sûreté Nucléaire (IRSN)), the official authority which estimates and evaluates the impact of nuclear installations, estimates that the latter represents less than 1% of the 2.9 mSv/year due to natural radioactivity to which, on average, each French person is exposed. The IRSN refers to this as negligible exposure. Nuclear facilities are also closely monitored. For example, at the la Hague site, nearly 50,000 environmental analyses are carried out each year. The impact of the plant is 100 times less than that of natural radioactivity. More than 50 years ago the nuclear industry developed technologies for recycling used fuel. By recycling nuclear material, we reduce the final volume of the most radioactive waste by a factor of five while packaging it in a safe manner (vitrification).
To find out more, see our ‘Unpacking nuclear’ item on: All about radioactive waste in France
- Understanding nuclear energy Nuclear energy makes it possible to produce low-carbon, competitive, and continuous electricity
- Nuclear power: a safe form of energy With over 50 years of nuclear industry experience in France, nuclear safety and protection are the sector's two absolute priorities.
- All about uranium Uranium is a metal ore that occurs naturally in the earth’s crust. Find out where it comes from, the difference between uranium 235 and 238, its uses in fuel, etc.