Potential Radioisotope-Element Harvesting from Recycling of Used Nuclear Fuel
AUTHORS
Sven Bader
Peter Henry
Orano Federal Services LLC
PRESENTED
March 13, 2024
Waste Management Symposia
INTRODUCTION
Radioisotopes are utilized in a variety of commercial sectors including nuclear medicine, space, and industry. In the nuclear medicine field, Orano Med is investigating the potential application of lead-212 for use as a targeted therapy against certain
cancers. Strontium-90 is similarly used in the medicinal field as it is utilized to generate yttrium-90 that is utilized for selective internal radiation therapy for liver and metastatic colon cancers. Strontium-90, as well as americium-241, have
been identified as potential heat sources for radioisotope power systems (RPS) that can be utilized in space and deep-sea exploration. Many existing space probes utilize plutonium-238; however, due to a limited supply of Pu-238, alternative radioisotopes
such as Am-241 are being sought. Krypton-85, with its unique characteristics as a noble gas, is utilized for air leak testing of space components, inspecting aircraft components, and hermeticity of semi-conductors.
Recent global events,
as well as the forecasted and existing demand for certain radioisotopes (e.g., Sr-90, Am-241, Kr-85) and elements (e.g., rare earth elements, noble elements) have brought about the need for an alternative, additional, and diversified supply chain.
In October 2023, Orano inaugurated the Stable Isotopes Laboratory (Laboratoire d’Isotopes Stables – LIS) on the Tricastin site in Southern France. This new facility is dedicated to the production of non-radioactive, stable isotopes to
meet the needs of health, research, and quantum sectors. This facility enables Orano to become a new alternative supplier in a growing market for these stable isotopes that is largely dominated by Russia, which currently produces nearly 70% of the
stable isotopes. Many radioactive isotopes utilized commercially are also sourced from Russia, and thus distributors of these radioactive isotopes and elements are seeking new sources. Orano has recently examined the feasibility of recovering specific
radioisotopes/elements from its La Hague used nuclear fuel (UNF) recycling facility in France.
While UNF awaits a final disposal path in the United States; in France, UNF is recycled at Orano’s La Hague facility, which contains two
of the world’s largest commercially operating UNF recycling facilities. Since 1976, the La Hague site has processed more than 40,000 tons of UNF. Orano’s UP2-800 and UP3 facilities separate usable uranium and plutonium from the fission
products generated during irradiation of the fuel in nuclear reactors. During this recycling process, noble and volatile fission gases (e.g., Kr-85 and I-129) are released from the fuel during the dissolution steps, while other fission products (e.g.,
Sr-90, Am-241) are collected in the raffinate stream of the aqueous separations process. While the fission gases are treated through an off-gas treatment process, the La Hague site does not currently capture krypton-85, and this gas is released through
the stack within regulated parameters. The fission products in the raffinate stream are concentrated and vitrified into a final borosilicate glass waste form that is suited for disposal as a robust, stable HLW form. The La Hague site presents itself
as a unique opportunity as an alternative source for radionuclides as many of these sought isotopes are currently treated as waste products.
KEY RADIOISOTOPES IN UNF
The potential for capturing radioisotopes in a used nuclear fuel (UNF) recycling facility is determined by a variety of factors. For an existing facility, such as La Hague, any capture process must be retrofitted into an operational facility with minimal
impact to ongoing operations and in accordance with its operating license. For new facilities, radioisotope harvesting operations may be considered during the design phase and may be better integrated into the facility, allowing for a potentially
larger number and quantity of radionuclides that may be efficiently recovered.
The types and quantities of radionuclides that may be recovered from UNF will vary depending upon quantity of UNF as well as the average burn-up, initial enrichment,
and cooling time of the UNF to be processed. In 2022, the La Hague plants processed approximately 920 MTHM of UNF (Ref. 1). In the near future, the anticipated throughput of the two plants may be conservatively assumed to be 1,000 MTHM/year. The burnup
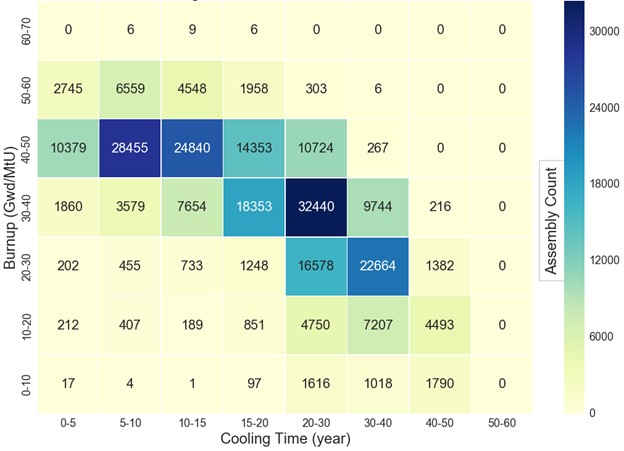
and cooling period of the fuel processed will also vary, but on average, the fuel may be assumed to have cooled for approximately 10 years. In comparison, Figure 1 shows the distribution of UNF in the United States against burnup (for fuel discharged
as of June 2013) and the average cooling age of the fuel is between 15 and 20 years and the average burnup is between 30-40 GWd/MTU.
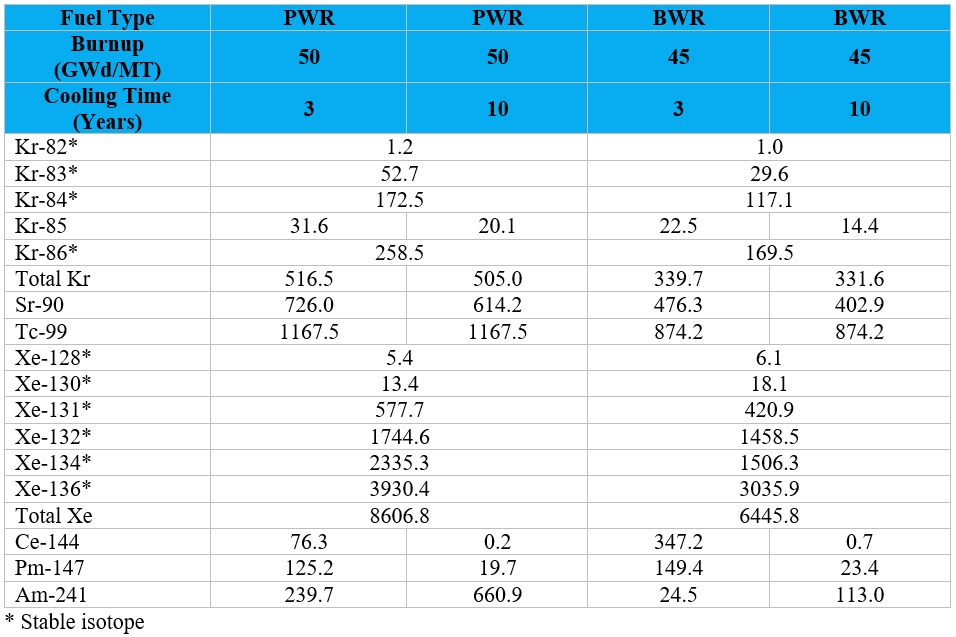
Table 1 provides an estimate of the total quantity of several radioisotopes and stable elements present
in UNF for various fuel types, burnup conditions, and cooling times. Note the Am-241 quantities actually increase over the cooling time as the Pu-241 in the fuel decays to Am-241, resulting in a buildup of Am-241. The rare earth element neodymium
can also be found in significant quantities in the UNF in quantities of up to 5,960 g/MTIHM1 after 3 years of cooling with all the short-lived radioactive radioisotopes of this element having decayed away after about 10 years, though
Nd-143, Nd-144, and Nd-150 remain slightly radioactive with half-lives of greater than 1.53 x 1011 per year. Another rare earth element, cerium, can also be found in significant quantities in the UNF in quantities of up to 3,590 g/MTIHM1 but of the 5 isotopes found initially at discharge in the UNF 4 are radioisotopes with half-lives of 1.4 days, 30.6 days, 284.9 days, and 5 x 1016 years and one is stable (Ce-140). Other rare earth elements present in UNF include lanthanum
(La, 1813 g/MTIHM1), praseodymium (Pr, 1649 g/MTIHM1), samarium (Sm, 1090 g/MTIHM1), yttrium (Y, 640 g/MTIHM1), europium (Eu, 225 g/MTIHM1), gadolinium (Gd, 224 g/MTIHM1), promethium (Pm, 125
g/MTIHM
1), terbium (Tb, 4.6 g/MTIHM1), and dysprosium (Dy, 1.5 g/MTIHM1).
1 After 3 years of cooling of PWR fuel burned to 50 GWd/MTU and an initial enrichment of 3.7 w/o.
OPPORTUNE LOCATIONS FOR RADIOISOTOPE HARVESTING AT LA HAGUE
These radioisotopes and elements are primarily found in two streams at the La Hague plant: within the dissolution off-gas stream and within the raffinate stream containing the fission products that are to be vitrified. Volatile and gaseous radionuclides (fission products, activation products, and impurities) are primarily desorbed during the shearing and dissolution steps at the head-end of the facility. As this off-gas stream contains radionuclides not found in similar concentrations as in other process off-gas streams for downstream equipment, this off-gas stream is handled independently from other process off-gases. The head-end off-gas treatment unit has several decontamination steps including (Ref. 3):
- Scrubbing columns for acid recovery that recombine and remove NOx from the off-gas streams.
- A caustic scrubbing column to remove iodine from the off-gas stream, transferring it to the liquid effluent process.
- The particulates of the purified off-gas are removed by high efficiency particulate air (HEPA) filters.
- Tritium is reduced through the cleansing columns and condensation.
- Residual iodine is removed by silver impregnated solid sorbents.
Non-volatile and non-gaseous fission products are found in the raffinate stream of the separations process of La Hague including Sr-90, Ce-144, and Am-241. The fission product raffinates are routed to the High Active Liquid Waste unit where the raffinates
are concentrated prior to being transferred to the vitrification process for their inclusion into a final borosilicate glass waste form. The majority of the radioisotopes and stable elements are found in this stream, which is highly radioactive and
thermally hot.
ISOTOPES LOCATED IN THE DISSOLVER OFF-GAS STREAM
Krypton-85
Krypton, including Kr-85, is not captured during the dissolver off-gas decontamination steps, rather all the krypton contained in the UNF is released at La Hague to the atmosphere through the facility stack after
having been mixed with other gas streams such as building ventilation. Orano measures and reports the quantities of gaseous radioactive exhausts released each year. For example, in 2022, Orano released 296,000 TBq/yr (8,000,000 Ci/yr) of noble gases,
including Kr-85, which was less than the limit set in ASN2015-DC-0536 of 470,000 TBq/yr (12,702,700 Ci/yr) (Ref. 1).
A Kr-85 capture process at the La Hague facilities would likely be implemented on the dissolver off-gas stream following
the decontamination steps, but prior to its mixture with other gas streams. Retrofitting the La Hague facilities to capture all of the released krypton gases is likely not feasible; however, the implementation of a smaller process that diverts a portion
of the bulk dissolver off-gas stream may be possible. Several methods to capture krypton have been and are continued to be studied, including cryogenic distillation, absorption, membrane permeation, and adsorption.
Cryogenic distillation
is utilized commercially to generate noble gases. Furthermore, a process for capturing Kr-85 has been operated previously at the Idaho Chemical Processing Plant (Ref. 4). This method of separation provides several challenges for its implementation,
though, as it requires a pre-treated off-gas stream that is free of water and NOx in order to prevent plugging of the equipment. In addition, any oxygen present in the line has the potential to form and accumulate as ozone, introducing
fire and explosion risks that are increased by irradiation from the Kr-85 (Ref. 5).
Adsorption is currently the most studied process for capturing noble gases from air. Adsorption utilizing zeolites has already been applied in the conventional
industry, while in the past two decades there has been a large volume of literature produced dedicated to Xe/Kr adsorption using MOFs (Metal Organic Frameworks). An adsorption process implemented on the La Hague off-gas system would likely involve
a multi-bed adsorption process similar to that proposed by PNNL in Ref. 6. After the initial off-gas pretreatment steps, stable xenon gases would first be removed, followed by a second column for the removal of the krypton gas. This captured krypton
would then be desorbed (either via pressure-swing or temperature-swing desorption) into a bottle for either further purification or shipment. The principal benefit of the MOFs is that they can operate closer to room temperature than other options.
Xenon
Due to the relatively short half-lives of the xenon radioisotopes, the cooling period of UNF treated at La Hague is sufficient to enable these isotopes to decay. However, the generation of xenon gas during
fuel irradiation will result in an increased Xe-136 abundance than in natural xenon. The valorization of Kr-85 from the La Hague facility may also enable the valorization of xenon as the xenon would likely need to be captured prior to the krypton
capture unit.
ISOTOPES IN THE RAFFINATE STREAM
Strontium-90
Strontium has previously been produced in large quantities in the U.S. through extraction processes. A similar separations process may be implemented on portions of the raffinate stream to generate a Sr-90 product.
The direct diversion of the raffinates stream to a process for the recovery of Sr-90 would be difficult due to its location in a highly active and inaccessible portion of the facility; however, alternative methods, such as the collection of raffinates
utilizing existing sampling methods may enable its capture. For example, liquid samples would be extracted in sample vials (~10 mL), such as one seen in Figure 2 that could be diverted to a hot cell where a laboratory scale extraction process could
be performed.

Figure 2. Sampling Vial
To implement a Sr-90 recovery process, several design and regulatory issues would need to be resolved. An optimal extraction, purification, concentration, and solidification process would need to be identified. Potential separation methods for strontium
include utilizing a solid media for adsorption (e.g., organic resins or minerals), precipitation (e.g., fuming nitric acid), or solvent extraction. An assessment of the selected process would need to be performed to ensure that orphan wastes are not
generated from the additional effluents that may result from the recovery process. Additionally, interim storage and transportation of the product, and potential ownership and regulatory concerns with French authorities would need to be considered.
Americium-241
Kilogram quantities of Am-241 are currently being sought by the space industry for their potential use in radioisotope power systems. In March of 2023, the National Nuclear Laboratory (U.K.) announced
a contract with the European Space Agency to supply this material (Ref. 7). While Am-241 may be found in the raffinates stream at La Hague, direct diversion and recovery of Am-241 from this stream involves the same challenges as those identified for
Sr-90, namely this stream is located in an inaccessible and highly radioactive portion of the plant. At La Hague, Am-241 is also found in canisters of plutonium placed in interim storage at La Hague prior to its shipment to the MELOX facility for
MOX fuel fabrication. This americium is generated from the radioactive decay of Pu-241 present in the plutonium cans. As an alternative to recovering the Am-241 from the raffinate stream, Am-241 could potentially be recovered from these cans of plutonium
oxide.
At La Hague, the UP2-800 facility includes a process for the redissolution of this plutonium in these cans should the isotopy of the stored material no longer meet the specifications required for MOX fuels by MELOX. These materials are
metered into the main separations processes for the plant and the plutonium recovered. This dissolved plutonium solution could be processed through additional steps to capture, purify, precipitate, filter, and calcine an americium oxide product. Similar
to the studies required for a Sr-90 capture process, the implementation of a Am-241 process must consider the impact of additional effluents produced (e.g., Ag from the Pu dissolver), interim storage, transportation, licensing, and the impact to ongoing
operations at the La Hague plant.
Noble Metals
UNF also contains a variety of valuable noble metals, such as molybdenum (Mo, 5,030 g/MTIHM1), ruthenium (Ru, 3,590 g/MTIHM1), rhodium (Rh,
771 g/MTIHM1), and palladium (Pd, 2,240 g/MTIHM1). These materials largely remain undissolved in the La Hague dissolver (undissolved solids, UDS) and are removed from the dissolver solution via a clarification step. These fines
are ultimately mixed with the high activity liquid waste concentrates in the vitrification area prior to being disposed of as high-level waste. Potentially, in a next-generation recycling plant, voloxidation may be used to remove and allow for the
recovery of some of the semi-volatile noble metal species (e.g., Ru). These metals would be deposited once they have cooled.
Methods have also been proposed for separating the noble metals from other fission products in the raffinate stream
and include:
- Separating Rh and Pd from alkaline Purex waste by liquid-liquid extraction with tricapryl mono-methyl ammonium chloride (Ref. 8).
- Recovery of Pd selectively from alkaline Purex waste by activated charcoal (Ref. 9).
- Separating noble metals from other fission products in nitric acid solution as a step of the process of removing the actinides from the high-level radioactive liquid waste by tributyl phosphate (TBP), then stripped by 9 M HNO3 (Ref. 10).
- Using lead oxide extraction as a method for recovering noble metals from fission product mixed oxides, which are obtained by calcining the HLLW (Ref. 11).
- Using Pb metal as a scavenger for noble metals in UDS and as a material to immobilize and store the radioactive noble metals (Ref. 12).
OPTIMIZATION OF NEW FACILITIES
The feasibility to implement radioisotope/element recovery activities in the existing La Hague facilities are limited by existing operations, design constraints, process flowsheets, and licensing regulations. These restrictions may be largely mitigated
in a newly designed reprocessing facility as the flowsheet and safety requirements may be designed with the intent of radioisotope/element harvesting. Implementation of these processes in a new facility would enable industrialization issues to be
considered in the initial design as opposed to as part of a retrofit design. Industrialization issues that must be addressed for radioisotope/element harvesting include: process monitoring and control, radiolysis, corrosion, solvent regeneration,
mass transfer/kinetics, reagent recycle, criticality control, and safety. This facility may further be equipped with processes designed to enhance radioisotope harvesting such as voloxidation and advanced partitioning.
Voloxidation is a
dry head-end process for the oxidation of UNF that may provide benefits such as the removal of tritium, the expansion of the uranium crystalline structure to facilitate dissolution, and the release of other fission gases (e.g., Kr-85) and volatiles
(e.g., I-129, C-14, Ru). By implementing a voloxidation process, gases targeted for harvesting are released in a more concentrated stream, potentially simplifying the design of the off-gas capture system.
A variety of separation processes
have been proposed in the literature for UNF recycling. Classically, tri-butyl phosphate (TBP) has been utilized as an extractant in a paraffin diluent (e.g., dodecane or hydrogenated tetrapropylene, HTP) with the goal of recovering U and Pu and rejecting
all other fission products and minor actinides to waste. Alternatively, newer solvents capable of extracting minor actinides are also being studied. Their primary goal is co-recovery of the transuranic actinides including the trivalent minor actinides.
They can also be designed to utilize simpler 1-cycle processes. Another goal is the CHON principle: using chemicals with molecules containing only carbon, hydrogen, oxygen, and nitrogen, resulting in an incinerable material without solids residue.
The processes may be designed to operate in the acidic versus high pH region.
POTENTIAL REVENUE AND BENEFITS
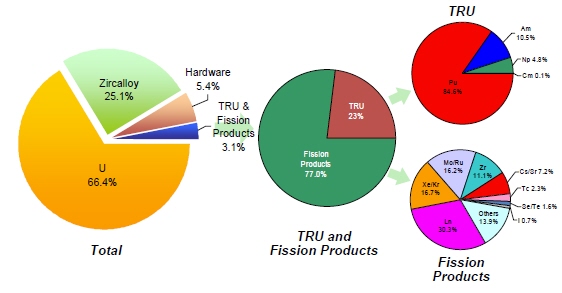
The level of effort to recover radioisotopes and stable isotopes/elements from the waste streams produced by a reprocessing plant recovering transuranics (TRU) is likely proportional to the revenue generated by, and the benefit associated with, their recovery and the complexity and cost of the recovery process(es). Figure 3 provides a breakdown of the makeup of UNF assembly illustrating the benefits of recovering:
- The U (66.4%) and the TRU (0.7%) for reuse in nuclear energy facilities.
- The zircalloy (25.1%) for reuse or minimally cleaning it up to LLW.
- Select radioisotopes and isotopes/elements from the fission products (2.4%).
By recovering the above products, the volume/weight of material to be disposed of as HLW is reduced and likely reducing the hazard of the wastes to be disposed (e.g., no criticality concern and no need for international safeguards, if applicable). If
implemented in the U.S., then this could also delay the need for a repository, but ultimately a repository will still be necessary.
According to a presentation made in March 2011 (Ref. 13), recovery of the zircalloy for a ~1000 MTIHM/yr
processing plant would result in a potential material cost savings in excess of $40M. Similarly, if the xenon gas is captured from the off-gases from the dissolver and shearing cells, then approximately $30M in revenue could be produced from the re-sale
of this collected gas. However, this needs to be balanced against the costs associated with re-designing of the off-gas treatment system, the operational and D&D wastes produced by the new collection unit, the impact to normal plant operations,
and the potential safety implications. Recovery of the noble metals could also produce a significant revenue stream estimated to be approximately $39M/yr for the ruthenium, $118.4M/yr for the rhodium, and $19.1M/yr for the palladium. These costs are
based on the worth of the element on the commercial market and do not consider the cost of the recovery process(es) and if in fact, the recovered isotopes/elements are eligible for commercial use (e.g., are the remaining isotopes all stable?).
To establish the value of each of the proposed materials to be recovered, a life cycle estimation needs to be considered for the approach planned to be implemented to recover this material from the process. Elements of the life cycle estimation
needed to be considered include:
- Need for a design, license, and building of a new process unit (consider space needed to install this process unit – is more building space or building modification required?)
- Inactive testing to demonstrate process works and can be effectively built (e.g., accounting for needed maintenance actions)
- Gaining regulatory and authorization to change the operational edict of the facility
- Need for new reagent(s) and associated space for reagent system
- Recycling or disposal of used reagents
- Safety impacts of implementing a new process unit
- Assessing impact of new process unit to operation of remaining portion of the plant
- Impact of inter-connections to other process units, including for off-gases
- Decontamination and deconstruction of process unit after use
- Revenue of product
- CAPEX and OPEX
- Benefits of removal of product from waste stream (e.g., volume reduction, increased waste loading)
The above assessment needs to be performed for either a new facility or a retrofit to an existing facility. Based on Orano’s recent experiences, costs to retrofit existing facilities can be expensive as interruption to the primary operation of the La Hague facility must be avoided and hence requires significant work arounds which must first be demonstrated in inactive testing facilities (e.g., at Orano’s HRB facility). In addition, another challenge is getting approval to modify the plant’s operating directive, which requires several layers of actions by La Hague personnel.
CONCLUSIONS
As noted, recent world events have pushed for alternative and diversified sources for the supply of select radioisotopes and stable isotopes/elements. Many of these materials are found in UNF and Orano is uniquely positioned to potentially provide these materials from UNF from its La Hague reprocessing facility, the largest commercial plants in the world. However, to collect these materials from an operating plant without interfering with the operation of the plant is quite the challenge and, although Orano has found a means to collect some of the requested materials from the plant with minimal interruption to its operation, the overall experience has been to defer incorporation of such recovery operations into a next generation facility (e.g., UP4). Some of this experience has been presented in this paper, but ultimately inclusion of advanced separations processes into new reprocessing facilities could benefit from a concerted effort to align and optimize recovery processes for these materials.
REFERENCES
- Orano Recyclage, “Rapport Annuel de Surveillance de l’Environnement Orano la Hague,” 2022. [Online]. Available: https://cdn.orano.group/orano/docs/default-source/orano-doc/groupe/ publications-reference/env-lahague-2022.pdf?sfvrsn=f89a9105_10
- L. MILLER, R. LEFEBVRE, G. RADULESCU, K. ROBB, et al. “Project: Spent Nuclear Fuel Characterization,” December 2016, ORNL, https://www.ornl.gov/division/rnsd/projects/spent-nuclear-fuel-characterization.
- M. VIALA, C. BERNARD, and P. MIQUEL, “Advanced PUREX Process for the New Reprocessing Plants in France and in Japan,” in Int. Conf. on Nucl. Fuel Reprocessing and Waste Manage., Sendai, Japan, Apr. 14-18, 1991.
- IAEA, “Separation, Storage, and Disposal of Krypton-85,” Vienna, Technical Report No. 199. 1980.
- P. BARON et al. “Treatment of Volatile Fission Products,” Nuclear Energy Agency, NEA/NSC/R(2022)4, Nov. 2022.
- J. LIU, C. A. FERNANDEZ, P. F. MARTIN, P. K. THALLAPALLY, D. M. STRACHAN, "A two-column method for the separation of Kr and Xe from process off-gases." Ind. & Eng. Chemistry Res., vol. 53, no. 32, pp. 12893-12899, Aug 2014, doi: 10.1021/ie502156h.
- National Nuclear Laboratory, “New contract from the European Space Agency to accelerate work on Americium-241.” nnl.co.uk. Mar 1, 2023.
https://www.nnl.co.uk/2023/03/new-contract-from-the-european-space-agency-to-accelerate-work-on-americium-241/ (accessed Nov. 9, 2023).
- M. CAMPBELL, Anal. Chem., Vol. 40, p. 6 (1968).
- J. PANESKO, ARH-1552, 1971.
- J. LILJENZIN, J. RYDBERG, and G. SKARNEMARK, Sep. Sci. Technology, Vol. 15, p. 799, 1980.
- G. JENSEN et al., Nuclear Technology, Vol. 65, p. 305 (1984).
- K. NAIO, T. MATSUI, and Y. TANAKA, “Recovery of Noble Metals from Insoluble Residue of Spent Fuel,” Journal of Nuclear Science and Technology, 23(6), pp. 540-549, June 1986.
- E. COLLINS, B. DELCUL, B. SPENCER, and B. JUBIN, “Recycle Update,” Separations/Waste Forms Working Group Meeting, Idaho Falls, ID, March 22, 2011.
>> Return to Orano White Papers
