Our activities
What is a stable isotope?
The matter (water, air, living organisms, etc.) that surrounds us is made up of atoms. Some atoms do not change. They are stable. Other atoms contain a surplus energy that they diffuse in the form of radiation. This is called radioactivity.
Atoms are made up of a nucleus surrounded by gravitating electrons. Inside this nucleus, there are neutrons and protons. It is the number of protons in the nucleus that gives it its identity.
Two different atoms may contain the same number of protons, but the number of neutrons is different. These are called isotopes. They belong to the same chemical element but have different properties.
In this case, we talk about isotope families. Stable isotopes do not emit radiation and therefore do not produce radioactivity. They have a wide range of applications, notably in healthcare, scientific research and industry.
In the 19th century, chemist Dmitri Mendeleev created a table to classify the families of atoms. This is how the periodic table of elements was created. 80 of the 118 elements comprising the periodic table have stable isotopes.
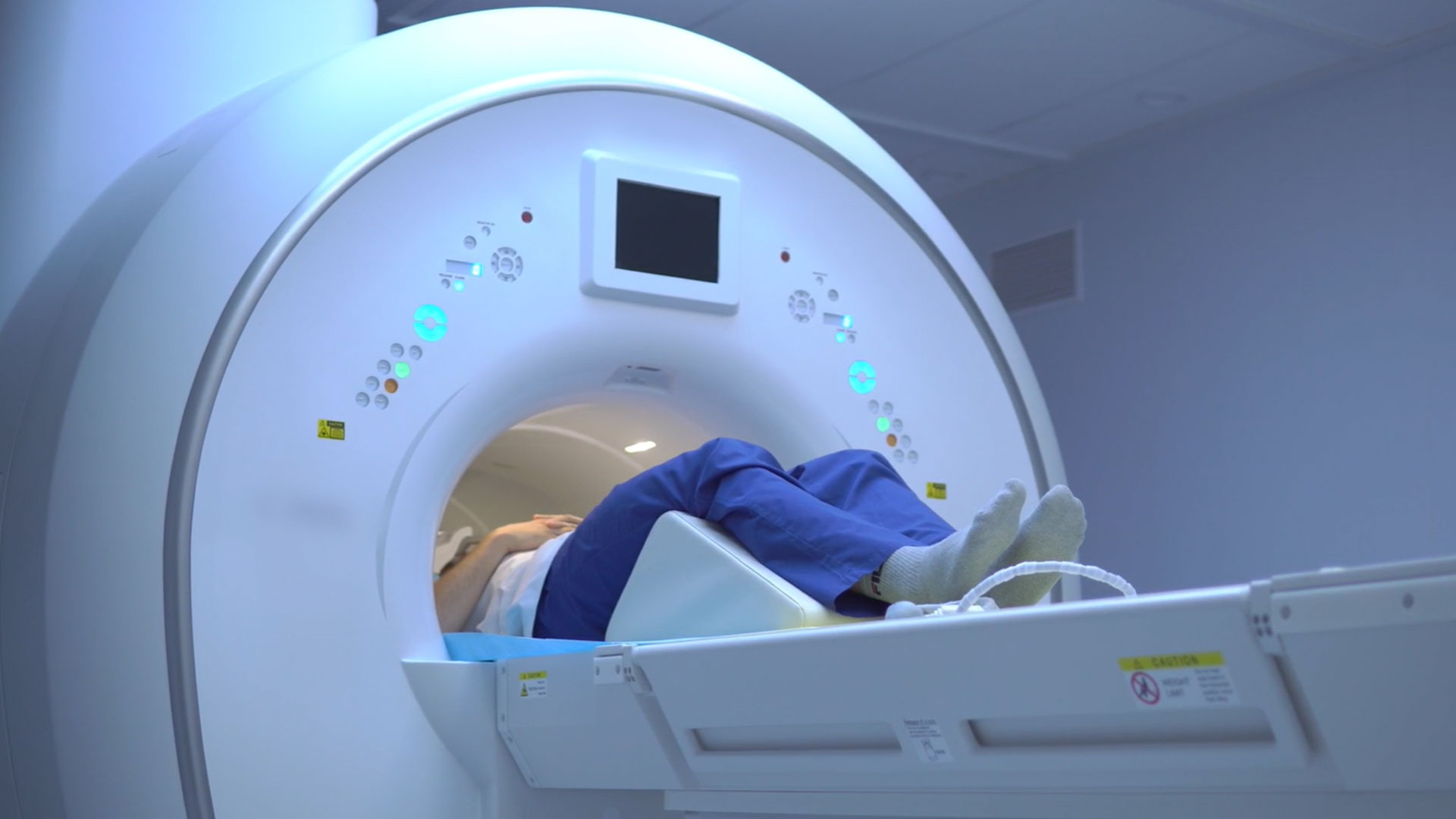
Healthcare
In the healthcare sector, stable isotopes are mainly used as a base for many radioactive drugs, thereby contributing to the diagnosis and treatment of cancer.
After purification and transformation by Orano, stable isotopes can be irradiated in research reactors, accelerators or cyclotrons in order to produce medical radioisotopes, which benefit nearly 35 million patients each year. Molybdenum isotopes can be used to make a solution of metastable Technicium 99, a radiation drug used in 80% of nuclear medicine procedures to perform a wide range of diagnostic operations, including lung scans.
In the case of isotope 100, which occurs naturally at 9%, transformation increases the percentage to over 99%. This makes it possible to obtain a material of high purity that meets medical requirements.
Similarly, stable isotopes can be used to improve magnetic resonance imaging (MRI) resolution. For example, Xenon-129, which occurs naturally at 26.4%, is used as a contrast agent for pulmonary MRI after purification and transformation.
Research
In basic research, stable isotopes are used in a number of promising areas such as quantum computing and fundamental experiments to improve our understanding of matter. For example, Xenon isotope 136 (8.9% in its natural state) is used for research on matter.
In the field of quantum computing, silicon-28 promises great advances in the industrialization of quantum chips with thousands or even millions of “qubits”. To do this, researchers need silicon enriched with isotope 28. Natural silicon comprises 92% isotope 28, which is increased to 99.9% after transformation.


Industry
In the industrial sector, the use of natural isotopes is growing, for example, to improve the performance of lasers. The use of stable isotopes such as zinc, depleted in zinc 64, also makes it possible to reduce the corrosion of cooling equipment in nuclear power generation reactors, while limiting the production of waste.
How does it work?
In its natural state, an element is made up of several isotopes. For example, carbon is composed of 98.93% carbon-12 and 1.07% carbon-13. The various users of stable isotopes require an element containing a very high percentage of a specific isotope, sometimes over 99.99%. The natural element therefore needs to be enriched. To achieve this, Orano uses centrifugation.
Centrifugation is based on the difference in mass between different isotopes. It consists of rotating a cylindrical bowl at very high speeds into which the element to be enriched is introduced in gaseous form. If the natural element cannot be in gaseous form at a temperature and pressure compatible with centrifuges, it must be converted to a compatible gaseous carrier (often a fluorinated element): this is the conversion stage.
Then, under the effect of centrifugal force, the heavier molecules of the element to be enriched are concentrated at the periphery while the lighter ones migrate towards the center. This basic stage of separating the molecules is repeated across a set of centrifuges placed in series and in parallel, an arrangement known as “cascade”. After this enrichment process, the gaseous element is transformed into the form required by the customer, either solid, liquid or gas.
[illustration: video animation - centrifuge]
During the first phase of developing the stable isotopes activity, Orano will use centrifuge enrichment technology. In a later phase, it plans to use other technologies to enrich stable isotopes, depending on market demand. This is possible thanks to the acquired know-how and modularity of the stable isotopes laboratory building.
Discover the centrifugation process

